About this module
Food safety is one of the most important aspects of human culture, and therefore it is essential in any regulatory framework for agricultural biotechnology. Fortunately, the risk analysis of foods derived from modern biotechnology is a highly harmonised discipline, due to the availability of specific Codex Alimentarius standards.
The work of risk assessors is very scientific in nature, but it also deals with legal/technical definitions. Codex guidance for the food safety assessment of products derived from “modern biotechnology” includes a definition of “modern biotechnology” to establish its intended scope.
It also states that such guidance may be useful for the assessment of foods improved by other biotechnology techniques, but this entails criteria to assess the applicability case by case.
A risk assessor, even a novice one, must know the legal and technical definitions that define the scope of their work. In most cases, the definitions will be equally applicable to most “GMO” or “transgenic” products currently on the market. Experienced regulators will have developed criteria for deciding if and how much the guidance is needed for other kind of products regulated in their country.
Major focus of the Module and main goals
The main goals of this module are to provide:
- A basic understanding of the risk analysis components applied to foods derived from recombinant-DNA plants, animals and microorganisms.
- Detailed theoretical knowledge on how to perform the safety assessment of this kind of foods.
- The opportunity to appreciate the practical aspects of safety assessment using case studies.
- Further introductory knowledge on particular aspects, such as allergenicity assessment, assessment of plants modified for nutritional or health benefits, assessment in situations of low level GMO presence, GMO labelling and detection methods.
- Background on the importance of Codex Alimentarius Guidelines, and familiarity with the guidelines applicable to the subjects covered in the module. Also, information on secondary sources of guidance is provided.
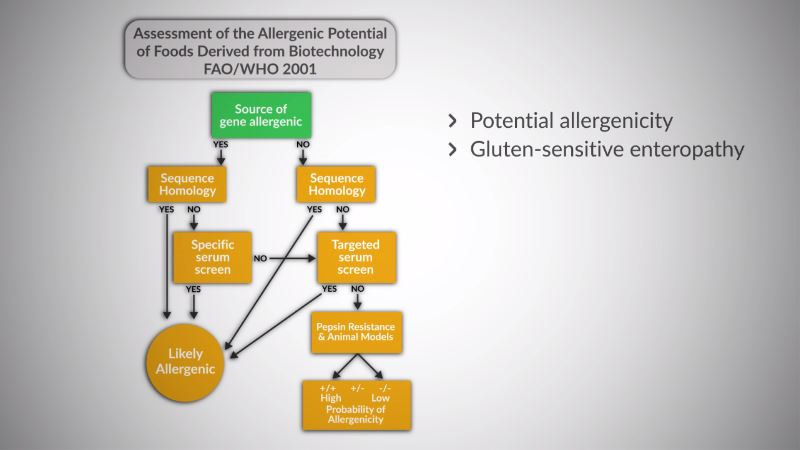
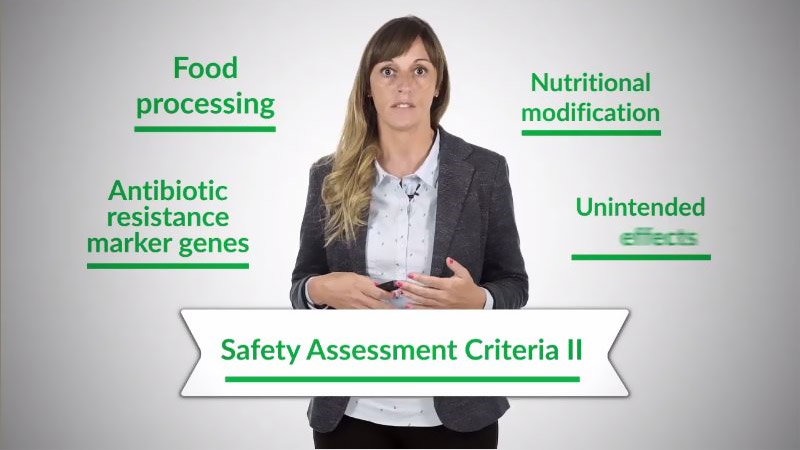
After completing this module, you will be able to manage the main regulatory aspects concerning this type of food. This includes having the ability to use relevant international guidelines, databases and scientific publications for these purposes.