About this module
The ERA is an essential part of the scientific and technical support and complements other components of such decision-making that will generally also include an assessment for the use of the GM crop as livestock feed and/or human food.
Major focus of the Module and main goals
The ERA of a GM plant encompasses a wide variety of disciplines from molecular biology, toxicology through to agronomy, botany and entomology. With such a wide scope of the ERA, it is essential to have a good grasp of how to manage the information load, use it effectively, and structure the inquiry in a way that the necessary concerns are addressed without the risk assessment straying into unproductive or unnecessary side issues. The focus of this module is to help the user to structure an ERA according to internationally accepted best practices and in such a way that the concerns identified in national regulations are addressed in a consistent and transparent manner.
Upon completing this module, you will be able to:
- Understand some of the key international concepts that have been developed for the ERA;
- Understand how to apply concepts such as familiarity to a comparative risk assessment, and how to use problem formulation to structure the ERA;
- Develop an understanding of how the ERA applies to each stage of environmental release, from developmental research through to full unconfined release;
- See how risk management measures can be applied post-release of the GM plant;
- See how an ERA was applied to support an actual commercial release.
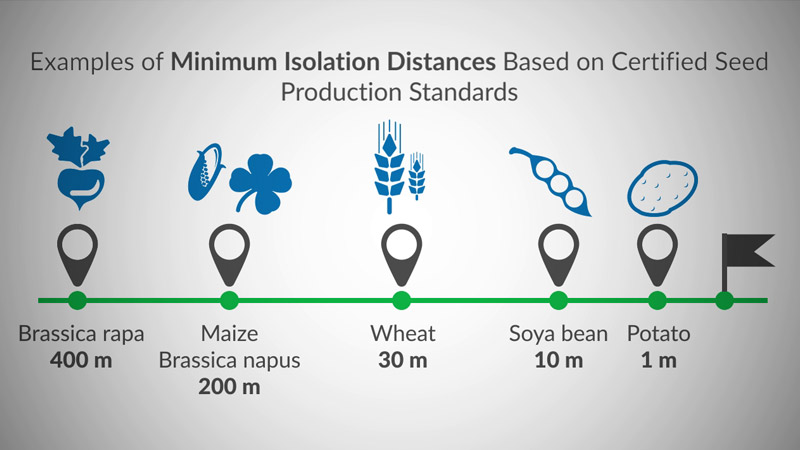
The ERA is fundamental to reaching a decision on every aspect of GM plant development from research stages, field trials to commercial product applications. The ERA is essential for assuring public confidence, so an understanding of how to conduct a scientifically sound, well-structured ERA is an integral part of any regulatory framework for biotechnology. This module will provide the user with practical experience and best practices.